Kinetics of adaptive immune responses after administering mRNA-Based COVID-19 vaccination in individuals with and without prior SARS-CoV-2 infections | BMC Infectious Diseases
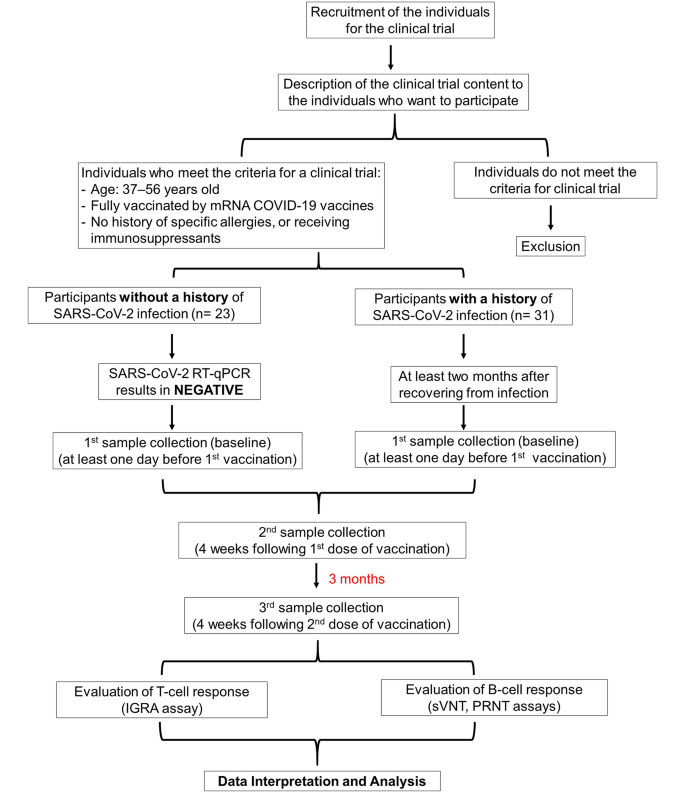
Cohort and study design
In total, 54 participants, 23 without and 31 with a history of COVID-19 at least two months prior (median 4, range 2–8 months) and who had received mRNA-based COVID-19 vaccines, were enrolled in this study (Fig. 1). The age range of the participants was 37–56 years old, with percentages of males 39.1% and 45.2% for uninfected and infected groups, respectively (Table 1). Among individuals in the uninfected group, 30.4% received PF and 69.6% received MO vaccination, while in the infected group, 40.6% received PF, and 50.0% received MO vaccination. Two participants (9.4%) in the infected group received mRNA-based COVID-19 vaccines; however, they could not recall which vaccine between PF or MO they had received (Table 1). Assessment of T- and B-cell responses to the specific SARS-CoV-2 antigen demonstrated a comparable level of immune responses between the PF and MO receivers in our previous study [17]. Moreover, considering the small sample size in our current study, classifying it into PF and MO groups would have been ineffective. Therefore, we combined PF and MO as an “mRNA-based COVID-19 vaccine”, and for further analysis, we focused our evaluation on uninfected and infected groups.
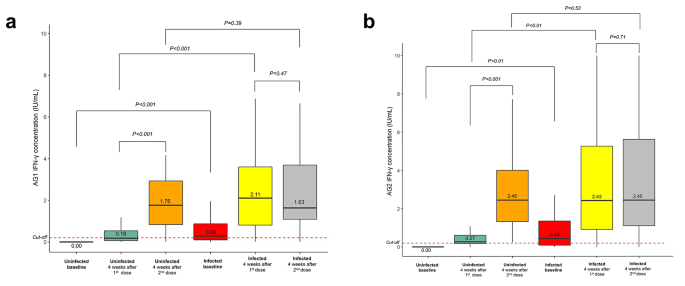
Vaccination in individuals with or without prior SARS-CoV-2 infections boosted the production of IFN-γ
After the first dose of the mRNA-based COVID-19 vaccines was administered, the QFN SARS-CoV-2 assay for assessment of the T-cell response to the SARS-CoV-2 specific antigen revealed a high positive rate of up to 47.8% and 92.3% for the uninfected and infected groups, respectively. In the baseline, the positivity was 0% and 58.1% for uninfected and infected groups, respectively (Table 2). After the second dose of the mRNA-based COVID-19 vaccines was administered, the positivity rate of the tested sample increased even more, up to 95.7% and 95.2% for the uninfected and infected groups, respectively (Table 2).
Administration of the first dose of mRNA-based COVID-19 vaccines increased IFN-γ in response to AG1 compared with the baseline up to a median 0.18 (interquartile range, IQR 0.07–0.57) IU/mL and 2.11 (IQR 0.80–3.63) IU/mL for uninfected and infected groups, respectively (Fig. 2a and Table S1). A similar pattern was also observed in the IFN-γ response to AG2 [median 0.27 (IQR 0.18–0.66) IU/mL and 2.43 (IQR 0.83–5.35) IU/mL] (Fig. 2b and Table S2). Following the administration of the second dose of mRNA-based COVID-19 vaccines, IFN-γ production in the uninfected group was significantly elevated compared with that after the first dose of vaccines, with a median of 1.76 (IQR 0.78–2.97) IU/mL, p < 0.001, whereas it declined slightly in the infected group [1.63 (IQR 1.09–3.69) IU/mL], p = 0.47, in response to AG1 stimulation (Fig. 2a and Table S1). Subsequently, in response to AG2 stimulation, the production of IFN-γ in the uninfected group was observed to be significantly higher than that after administration of the first dose of vaccine [median 2.45 (IQR 1.20–4.10) IU/mL, p < 0.001]. Meanwhile, in the infected group, the difference in IFN-γ production in response to AG2 stimulation after the second dose of vaccination was not significant [median 2.45 (IQR 1.11–5.62) IU/mL, p = 0.71] compared to that after the first dose (Fig. 2b and Table S2).
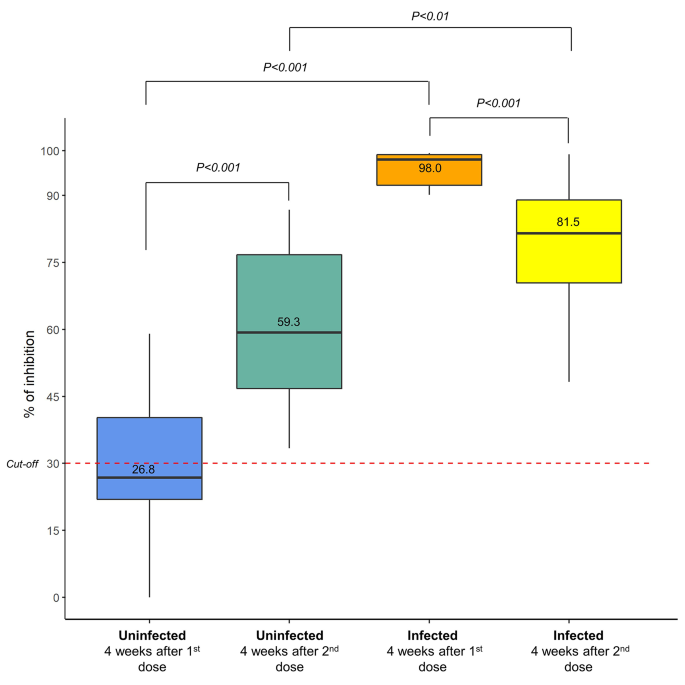
Neutralizing antibodies are generated in uninfected and infected groups after administering mRNA-based COVID-19 vaccines
Evaluation of the neutralization antibody by the cPass sVNT assay demonstrated a high positivity rate after the mRNA-based COVID-19 vaccination. The positivity rate after the first dose of vaccines was up to 95.7% and up to 100% for the uninfected and infected groups, respectively. Subsequently, after administering the second dose, the positivity rate for both the uninfected and infected groups reached 100% (Table 3).
Assessment of the neutralizing antibody by cPass sVNT showed that vaccination with mRNA-based COVID-19 vaccines triggered high inhibition in individuals with or without prior SARS-CoV-2 infections. In the preliminary experiment, we found that all tested sera yielded an exceedingly high percentage of inhibition (close to 100%); hence, comparing the percentage of inhibition among the groups using this result would have been ineffective. Using the sera that were diluted up to a ratio of 1:20, we found that the percentage of inhibition after administering the first dose of mRNA-based COVID-19 vaccines in the infected group was higher than that in the uninfected group: median 98.0 (IQR 91.5–99.1) % and 26.8 (IQR 20.0-40.5) % (p < 0.001), respectively (Fig. 3 and Table S3). A similar pattern was also observed after administering the second dose of mRNA-based COVID-19 vaccines, where the median percentage of inhibition was 81.5 (IQR 70.4–89.0) % and 59.3 (IQR 46.6–76.8) % (p < 0.01) for infected and uninfected groups, respectively (Fig. 3 and Table S3).
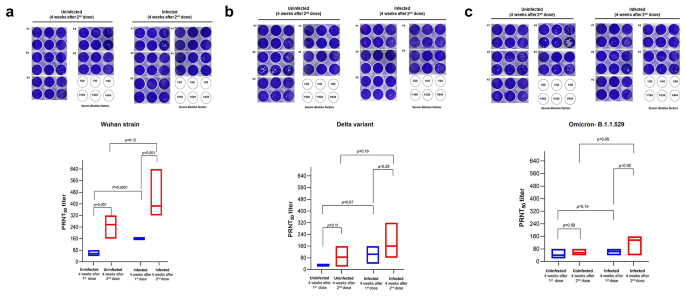
Neutralizing responses vary against the original strain of SARS-CoV-2 and the variants of concern
We measured the neutralization efficiency of the neutralizing antibody from the serum samples against SARS-CoV-2 Wuhan strain, Delta, and Omicron- B.1.1.529 variants by PRNT50 assay. Five serum samples from each uninfected and infected group were selected and diluted from 1:20 to 1:640 before being subjected to the PRNT50 assay. Our assessment demonstrated that PRNT50 titers increased over the vaccine doses.
After administering the first dose of mRNA-based COVID-19 vaccine, the serum from the uninfected group was able to neutralize SARS-CoV-2 variants at a median dilution of 1:40, with the lowest dilution of 1:20 and the highest of 1:80. Meanwhile, the infected group appeared to have at least twofold higher PRNT50 titers, where the serum was able to neutralize SARS-CoV-2 variants at a median dilution of 1:80, with a range of dilution factors of 1:40 to 1:160. The serum’s ability from uninfected and infected groups to neutralize the virus was significantly higher after administering the second dose of vaccines than after administering the first dose. Nevertheless, the PRNT50 titer was observed to be higher in the infected group than in the uninfected group with the ability of serum to neutralize the SARS-CoV-2 variants at a median dilution factor of 1:160, with the lowest dilution factor of 1:40 and the highest of 1:640 (Fig. 4). In response to the variants of concern, the serum samples after administering the second dose of vaccines, either from the uninfected or infected group, demonstrated a high neutralizing ability to the Wuhan strains but appeared to be less effective against Delta or Omicron-B.1.1.529 variants [median dilution factors of 1:320, 1:160, and 1:160 for Wuhan strain, Delta, and Omicron-B.1.1.529 variants, respectively] (Fig. 4).
Neutralizing activity in individuals with or without prior SARS-CoV-2 infections. (a) representing neutralizing activity against the Wuhan strain, (b) against the Delta variant, and (c) against the Omicron variant. The upper panel represents the plaque reduction from each serum dilution. The lower panel demonstrates the PRNT50 titer